Global Health Working Group
See more information here
TMRP Global Health Pump Priming Awards
1. Photovoice to explore community members perspectives regarding health and healthcare challenges in Mukono District, Uganda
PI: James O'Donovan and David Musoke, Makerere University School of Public Health
There have been calls for a greater number of clinical studies in low- and middle-income countries to explore locally identified areas of concern, ensuring they are contextually relevant. One useful approach towards this is Community Based Participatory Research (CBPR), which broadly involves local stakeholders being central to the research process. CBPR is often guided by the hypothesis that such an approach can help to ensure research remains contextually relevant and better understand the lived experiences of a community. Specific to trials, CBPR has consistently been suggested as a means of diversifying participation and increasing relevance and quality to end users (i.e., patients or community members). One method aligned to CBPR is photovoice, whereby cameras are given to individuals to capture photographic images around a central theme of community importance or concern. In our project, the subject of interest is concerns and challenges related to healthcare in the community. To date 15 community members from the Seeta Nazigo Parish, Mukono District in central Uganda have been selected to participate. They have been trained in the use of cameras and have undergone one round of photographic capture and image discussion with the project team.





2. Pilot implementation of Short Message Service for randomisation in a multisite pragmatic factorial clinical trial in Kenya (PRISMS Study)
PI: Mercy Chepkirui Terer, KEMRI-Wellcome Trust Research Programme, Nairobi, Kenya
Failure to achieve valid randomisation and allocation concealment may result in biased estimates of treatment effects and potential loss of objectivity in trials. The traditional use of envelopes is vulnerable to manipulation and risk of damage to envelopes during shipping and storage, while filling and sealing envelopes is a time-consuming process prone to error. Web-based randomization methods are preferred; however they are expensive and not suitable in low resource settings with poor internet connection. Mobile-based text messaging offers a low-cost and reliable alternative. PRISMS is a study that aims to design, develop and pilot a short message service (SMS) randomization platform for trials in resource-limited settings versus sealed, opaque envelopes within an ongoing randomized controlled trial. The platform has 3-tiers: (1) data tier, hosting platform data, (2) business logic tier, managing application transactions (3) the presentation tier, consisting of the SMS interfacing, administrative dashboard, and Android application. An evaluation will be conducted to determine random sequence accuracy, document user experience, and measure response time and cost. A user framework will be developed to guide implementation at scale in future studies and to provide a reliable and low-cost alternative platform to support the increasing number of large and complex clinical trials in low-resource settings. We used open-source tools to design the application architecture workflow diagrams and the database schema, have completed the business logic and data layers, the administrative dashboard and the SMS interface, and there is a functional prototype for field testing. Dummy randomization lists were used to test the randomization process with dummy trial sites with results indicating an SMS average latency rate of 00:38 seconds with the fastest delivery in 00:13 seconds with an allocation accuracy of 100%. Over the next three months, we will develop an Android application to complement the SMS interface with Phase 2 resuming once trial field activities resume.
3. Cultural competence in trial design and conduct
PI: Dr Nandi Siegfried, South African Medical Research Council
This project aims to evaluate applicability and utility of currently available tools to measure cultural competence in trial design and conduct. Cultural competency refers to consideration of the cultural and linguistic diversity of the populations targeted for inclusion into a trial. Failure to consider relevant cultural and diversity parameters during trial protocol development and trial conduct may negatively impact recruitment, intervention development and delivery, adherence, and retention, potentially reducing overall internal validity. We will retrospectively apply the 2007 Gibbs framework and the GRIPP-2 checklist to Project Mind, a three-arm cluster randomised controlled trial of 1,340 patients in 24 primary care clinics in urban and rural settings in South Africa. Project Mind compares two different systems approaches to integrating mental health counselling into chronic disease care with treatment as usual. To date, we have completed secondary data source identification and collation, pilot tested the Gibbs framework on trial documentation, presented the methods in a journal club and received feedback, and created an interactive trial timeline.
4. Exploring barriers to data reuse
PI: Naomi Waithira, Mahidol Oxford Research Unit
Despite the effort and investment to promote data sharing in the last decade, secondary data users still cite lack of access to quality and relevant data as a challenge. Ironically, repositories report low usage of clinical research datasets. This study aims to establish if and how clinical research datasets existing in the public domain are accessed and used and to describe barriers and opportunities to promote use of shared data from the perspective of secondary data users. With a focus on respondents from Low- and Middle-Income Countries (LMICs), we will conduct an online survey among clinical researchers and in-depth interviews with individuals who use clinical research datasets. Results will be valuable in identifying which interventions should be prioritised to increase utilization of shared datasets in LMICs. We anticipate increased reuse of data will result in improved quality and transparency in science, improved public health and patient outcomes, and better return on investment in research. The study is approved by the Oxford Tropical Research Ethics Committee, data collection tools have been developed and we anticipate beginning data collection in February 2021 with ethical approval for participation of investigators from Kenya in April 2021.
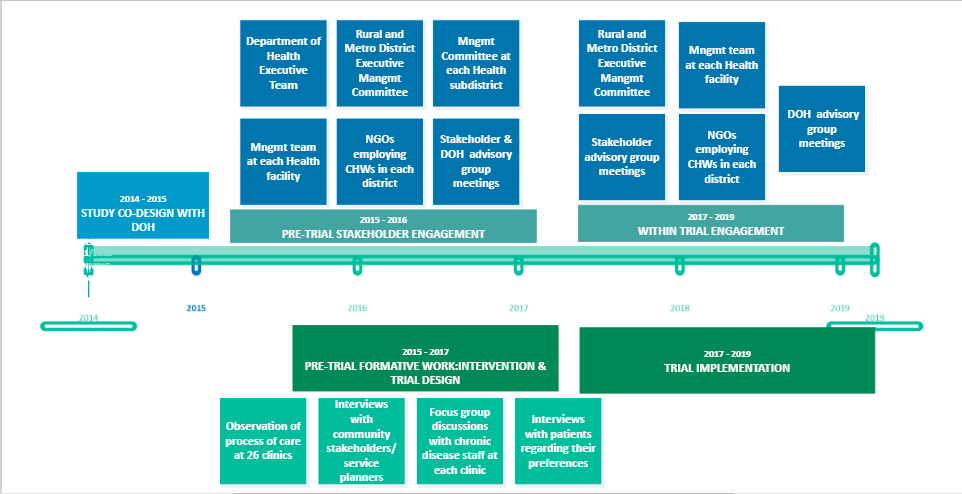
5. The practice of pilot/feasibility studies in informing the conduct of HIV related clinical trials in sub Saharan Africa
PI: Dr. Sylivia Nalubega, Soroti University
Pilot/feasibility studies represent a fundamental phase of the research process and play a vital role in the preliminary planning of a full-size trial. Sub-Saharan Africa hosts most HIV trials in the world, however, it is not well documented how pilot/feasibility studies are utilised as a pre-requisite step for the conduct of larger HIV trials in this region.The objective of our scoping review is to establish the extent to which larger HIV related trials in sub-Saharan Africa are informed by a prior pilot/feasibility study. We are following the JBI methodology for scoping reviews. Six databases including MEDLINE (OVID), CINAHL, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL) databases, and African Index Medicus (AIM) were systematically searched to identify potential articles, as was the grey literature. Data extracted and charted using a structured tool adapted from the JBI methodology will now be analysed and interpreted using simple descriptive statistics. Patterns and trends (if identified) will be illustrated using figures and/or diagrams and summarized in a narrative. Final conclusions will then be drawn and recommendations for research and practice proposed.
6. Optimising Informed CONsent in clinical trials in low- and middle-income settings: feasibility of an adapted QuinteT Recruitment Intervention (QRI) in India (OrION-I)
PI: Dr Sangeetha Paramasivan, University of Bristol
This study at King Edward Memorial Hospital-Mumbai aims to investigate the feasibility and acceptability of audio-recording clinical trial discussions with patients, analysing them qualitatively and using them to provide feedback to recruiters to optimise informed consent. Methods employed will involve audio-recording trial consultations, interviewing patients and recruiters to elicit their perceptions regarding the audio-recording and feedback processes, and developing data management/sharing mechanisms for a large-scale study. The methods draw from the QuinteT Recruitment Intervention (QRI), developed at UoB with support from MRC-HTMR and applied in nearly 70 trials in the UK, to optimise recruitment while safeguarding informed consent. OrION-I study team’s longer-term vision is that the research at KEMH-Mumbai, which is a large, research-active, tertiary referral centre in India, will facilitate the development of a collaborative grant aimed at optimising informed consent in clinical trials across other centres in India. Despite some early delays due to the pandemic, we have been able to make considerable progress in obtaining approvals and are now at the stage of final approval required to start data collection.



7. Assessment of the challenges encountered in implementing vaccine clinical trial methodologies in Tanzania
PI: Wigilya P. Mikomangwa, Muhimbili University of Health and Allied Sciences
Effective vaccine use is a global approach expected to eliminate infectious diseases. Several vaccines are in various phases of trials for Tuberculosis (TB), malaria and human immunodeficiency virus (HIV). However, only a few vaccine clinical trials are conducted in low- and middle-income countries, including sub-Saharan Africa. To improve this situation, we are conducting an exploratory qualitative study in vaccine clinical trials at Muhimbili University of Health and Allied Sciences (MUHAS) and Ifakara Health Institute (IHI), Tanzania to explore the challenges encountered by investigators when implementing vaccine trial methodologies. Key informant interviews are being conducted with trial coordinators, principal investigators, pharmacists, nurses and laboratory scientists, and also trial regulators and ethical committee members to explore their experiences. Trial participants are also invited to focus group discussion to explore the challenges they may encounter.

Publications
Nalubega S, Osuwat LO, Agyeiwaa PB, Evans C, Matovu JB. The practice of pilot/feasibility studies in informing the conduct of HIV related clinical trials in sub-Saharan Africa: A scoping review. Contemp Clin Trials Commun. 2022 Jul 8;29:100959. doi: 10.1016/j.conctc.2022.100959. PMID: 35865280; PMCID: PMC9294242.
Siegfried NL, Hopewell S, Erasmus-Claassen LA, Myers B. Evaluation of cultural competency in a South African cluster randomised controlled trial: lessons learned for trial reporting standards. Trials. 2022 Oct 29;23(1):918. doi: 10.1186/s13063-022-06767-y. PMID: 36309756; PMCID: PMC9617747.
Allen E, Wang D, de la Horra A, Chepkirui Terer M, Mikomangwa W, Musoke D, Nalubega S, O’Donovan J, Paramasivan S, Siegfried N, Waithira N, Williamson P, Lang T. The Trials Methodology Research Partnership; A global community of practice for improving design, conduct, & analysis of trials everywhere. Poster & oral presentation at the EDCTP Forum. Maputo, Mozambique. Oct 2021.










